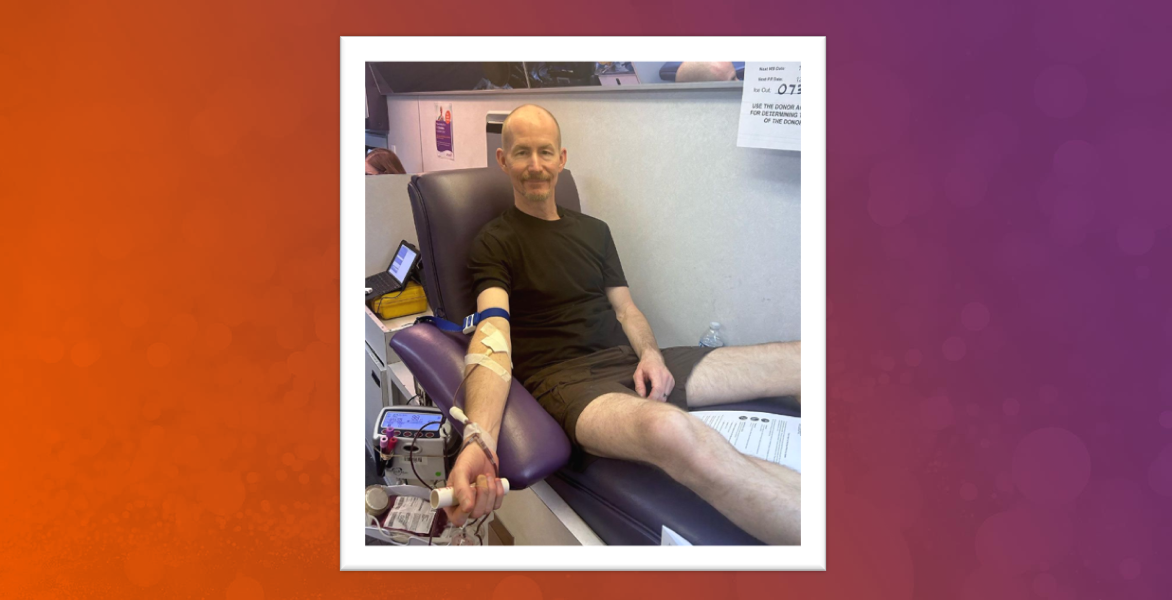
Now newly eligible, Mark and his husband Ken came forward to give blood together and celebrate this landmark change in donor eligibility. Together for about 26 years, they were previously ineligible to give due to the FDA’s former Men Who Have Sex with Men (MSM) guidance which required gay and bisexual men to wait three months following their last sexual contact with another man.
As part of their donation, Mark and Ken underwent the individual risk assessment, which included questions about their recent behaviors and potential risk factors. Like all donors, they were evaluated for their eligibility based on objective criteria, ensuring the safety of the blood supply.
“We are both happy to be eligible to donate blood after all these years,” said Mark, who has worked at Vitalant and in the blood banking industry for most of his career. “I had never donated, and Ken hasn’t donated since high school. I’m thankful for the chance to help save lives.”
Mark and Ken emphasize their gratefulness for the opportunity to donate and encourage others who may have been previously deferred to check their eligibility and schedule a blood donation to support patients in need.